Humans have always turned to nature for tips, tools and #inspo.
For centuries, we’ve used plant and animal pigments to dye our clothes all the colours of the rainbow.
But some shades come easier than others.
THROW SOME SHADE
In nature, green or blue colourants are tricky to make.
Nowadays, we can create blue things in two shakes of a dog’s tail. But before synthetic dyes, plant-derived indigo was ‘blue gold’, a commodity so valuable that many people were exploited in its production.
Equally tricky to source were green dyes. Mostly, people would mix indigo with yellow pigments from saffron, turmeric and onion skins.
However the small hairstreak butterfly—like many other butterflies—has been able to avoid the chemical route altogether. To get its Grinchy hue, it simply physically mimics the wavelength of light.

SEEING GREEN
So the dogma is light travels in waves.
Different colours correspond to different wavelengths. Wavelengths are measured by the distances between peaks and troughs in light waves.
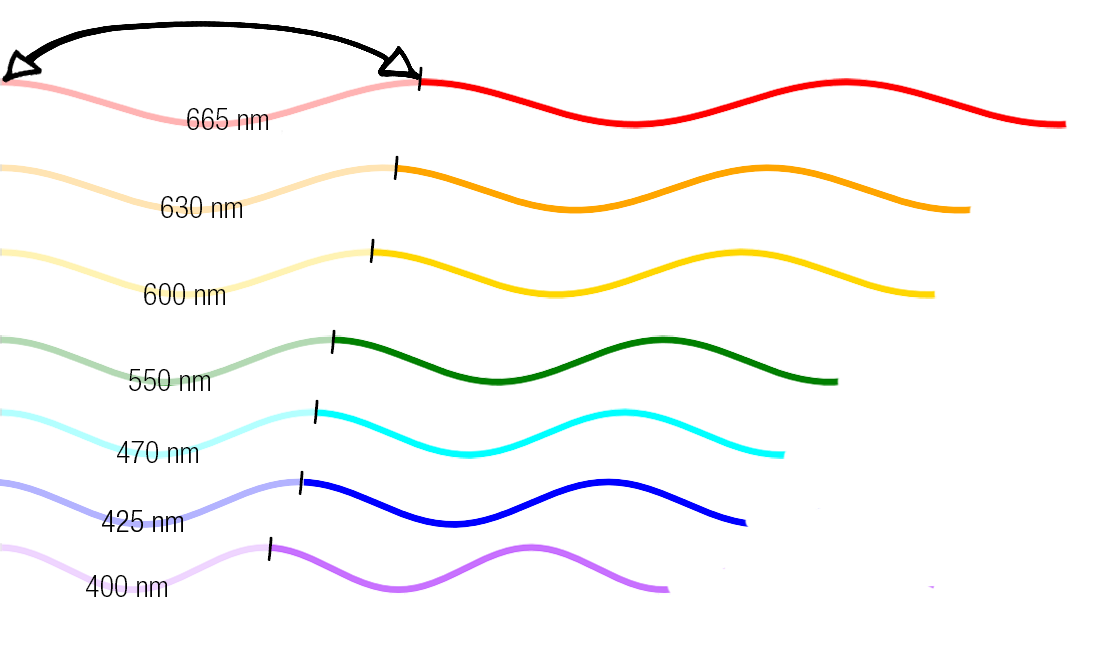
We perceive things to be certain colours because pigments absorb certain wavelengths.
My jeans are blue because they contain pigments that absorb violet, indigo, green, yellow, orange and red light but reflect blue. My shoes are black because the leather was treated with stains that absorb all colours, and my shirt is pink because it’s just a damn cute colour.
LET’S GET PHYSICAL
But colour is not always chemical. Sometimes it’s physical.
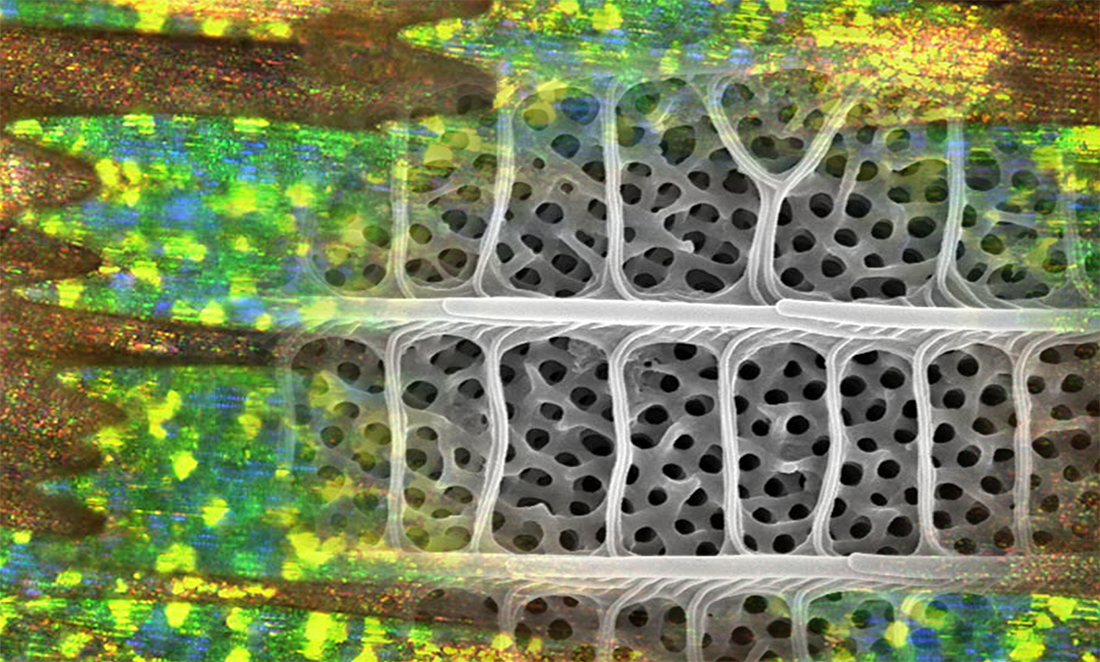
On the hairstreak’s wing, structural colouration occurs when light bounces off microscopic crystallites.
The crystallites have this crazy 3D labyrinth structure. Scientists call them gyroids.
A gyroid nanostructure network covers individual scales on the wing. Crystallites run up ridges along the scales and are crossed by ribs.
This means that each individual butterfly scale is covered by a complex but highly regular structure with evenly spaced peaks and troughs.
Because the distances between peaks and troughs of this structure match the wavelength of green light, we see green.
THE HARD STUFF
Biological gyroid nanostructures have only been thoroughly studied quite recently. But not because scientists weren’t interested in them.
Their really, really ridiculously tiny size makes them pretty tricky to examine. Literally, a centre for ants would be a thousand times too big for them.
Another problem is that most of them are made from a thin membrane supported by water.
To try and get a glimpse of these living structures inside an electron microscope, we have to put them in a vacuum.
This goes about as well as blowing soap bubbles in outer space—in other words, not well.
Without any air to push back down on the membrane, they burst. Quickly.
But our butterfly’s gyroids are not made from membranes. Rather, they’re made from a hard material called chitin. It’s a sugar that is found in the shells of insects and crustaceans as well as in fish scales and mushrooms.
And it’s significantly easier to get a good picture of what’s under a microscope at the nanometre scale.
TINY INSIGHTS
Nanostructures are pretty much everywhere, and they’re useful for just about everything.
They make lotus leaves self-cleaning. They make gecko feet sticky. They help water striders walk on water.
We can only observe the ones that create an optical effect, but even then, they’re pretty common.
The lustrous rainbows that play on oyster shells at different angles. The vibrant hues of the (innovatively named) blue-and-yellow macaw. Or the marble berry, which might be the brightest biological material in the world.
All of these come from nano bits and bobs interfering with light.
Even among butterflies, nanostructures are common. They can create blues, greens and iridescence. Even the anti-reflection coating on near-invisible glasswing butterflies have nanostructures to thank.
Basically, the hairstreak butterfly’s gyroids are special—but not that special.
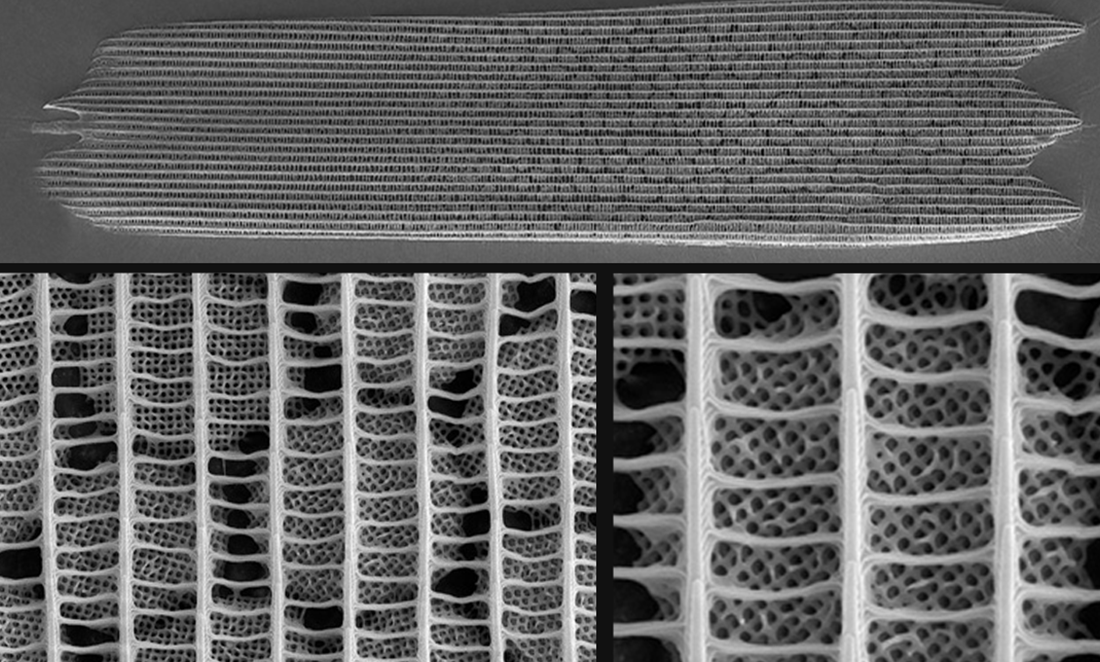
What does make it unique is that, for the first time, we’ve got a picture of how the nanostructures might form.
A global collaboration—including Murdoch University researchers—have described what looks like growing gyroids marching up from the root to the tip of wing scales.

Like the von Trapps lined up in their matchy-matchy uniforms, the nanocrystalline structures progress from little to big.
From this snapshot, scientists can infer how nanostructures come to be.
GREEN-EYED MONSTER
All of this nano action makes scientists a little jealous.
Humans could use nanostructures for so many different and useful things.
And we do use a lot already in our everyday lives. But we’ve only been studying the things for the last few years. Nature has a tiny head start (read: 3 billion years) in evolving efficient mass production of nanostructures.
So how do butterflies frost themselves in nano bling at the bat of an ommatidia? This observation of the hairstreak’s wing is the first step in answering that question.
But surely before we know it, our clothes will be covered in self-cleaning, colour-changing, climate-controlling invisible nanostructures.
It’s not the first time humans have taken inspiration from nature. And it sure won’t be the last.