Calling something a Gordian knot is to say it’s an unsolvable problem. It comes from a story of Alexander the Great, who encountered the famous knot near Turkey.
The person who untied the impossible knot was prophesied to conquer all Asia.
Alexander, trying for a while, got fed up and cut the knot with his sword. He used his army to conquer parts of Asia instead.
But this was just a bit of rope. What would a true impossible knot look like?
Well, scientists partnered around the world are tying them and they’re much too small to cut with a sword.

An atom is a single particle from one of the periodic table’s elements. Multiple atoms can join to form molecules. Molecules make up the world around us.
Even if two molecules have the same atoms, the way they’re arranged can vastly change their properties.
In fact, the structure of molecules with the same atoms is so important it has its own discipline.
Chemical topology studies how molecules can be twisted, stretched and deformed without breaking them.
Life is a knotty thing
Chemistry Professor George Koutsantonis at the University of Western Australia explains why topology has researchers all tied up.
“People are interested in complex topologies because you are one. Human beings evolved from primordial soup into complex architectures, and we want to try and explain that.”
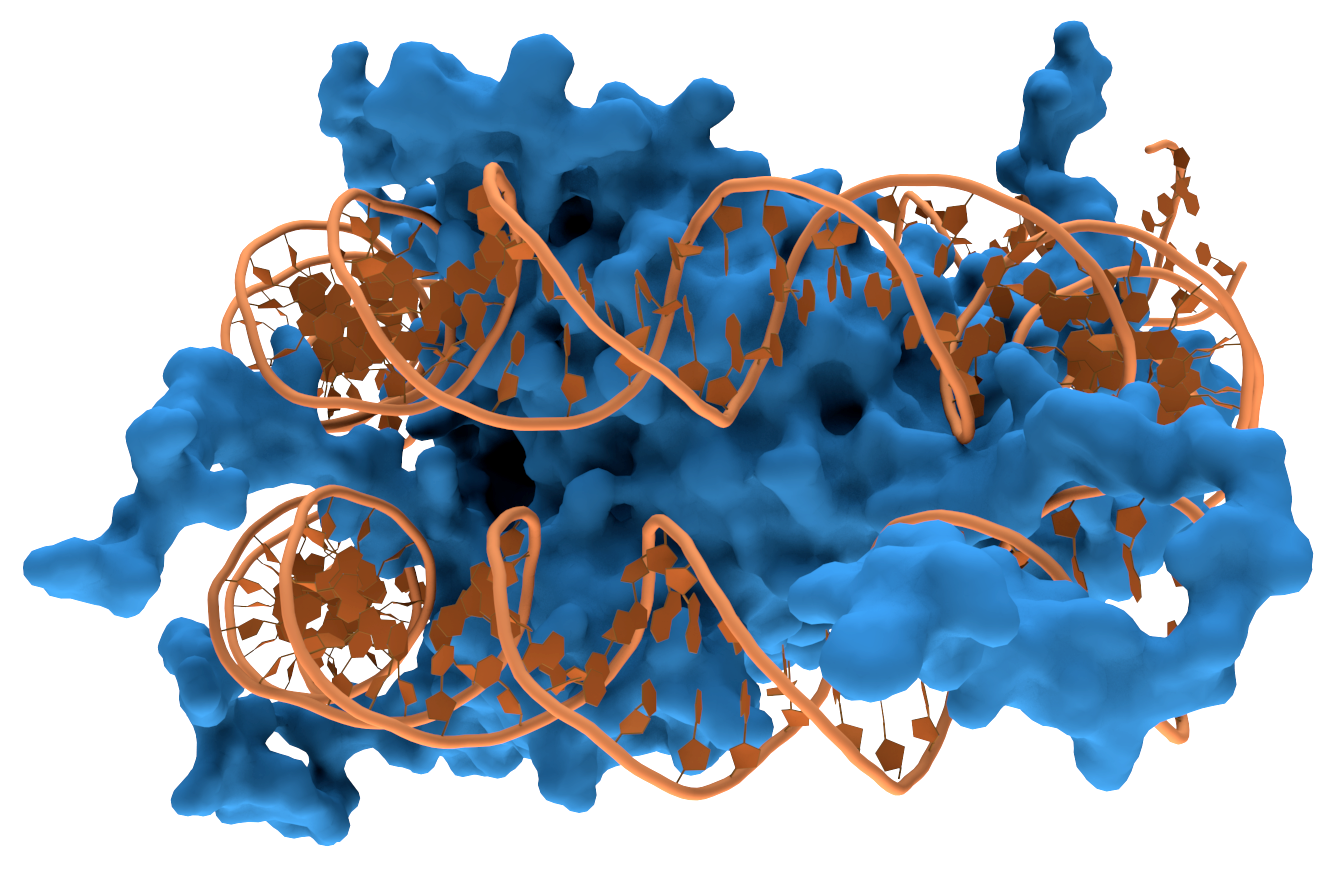
Knots are vital for DNA, where they compact and store the DNA until proteins unwind it to create messages or new cells.
Certain molecules always appear to have a left or right-handedness to their knots.
Amino acids are always left-handed, sugars and DNA are right-handed. Why every living cell on Earth needs to tie its knots a certain way is an ongoing mystery.
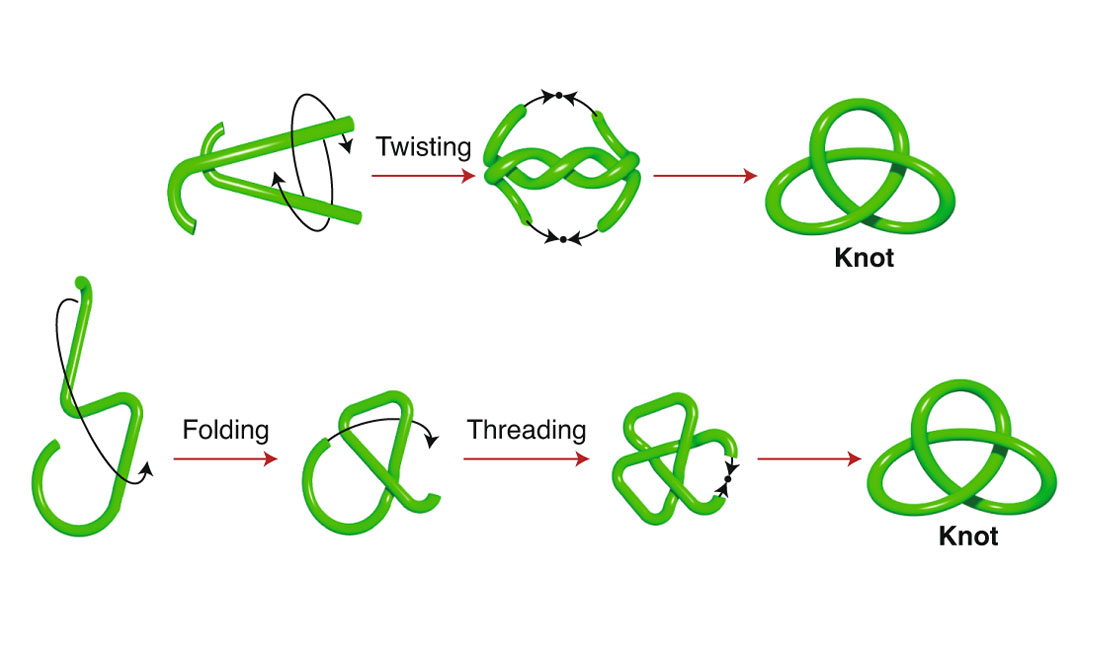
Which brings us to tying a knot. The best way to learn how molecular knots behave is to tie them.
Opposites attract
It starts with some metal atoms.
“Up until 60 years ago, researchers were focusing on biological chemistry. Then it became apparent that biological systems use metal ions, like your brain neurons need sodium and potassium ions,” says George.
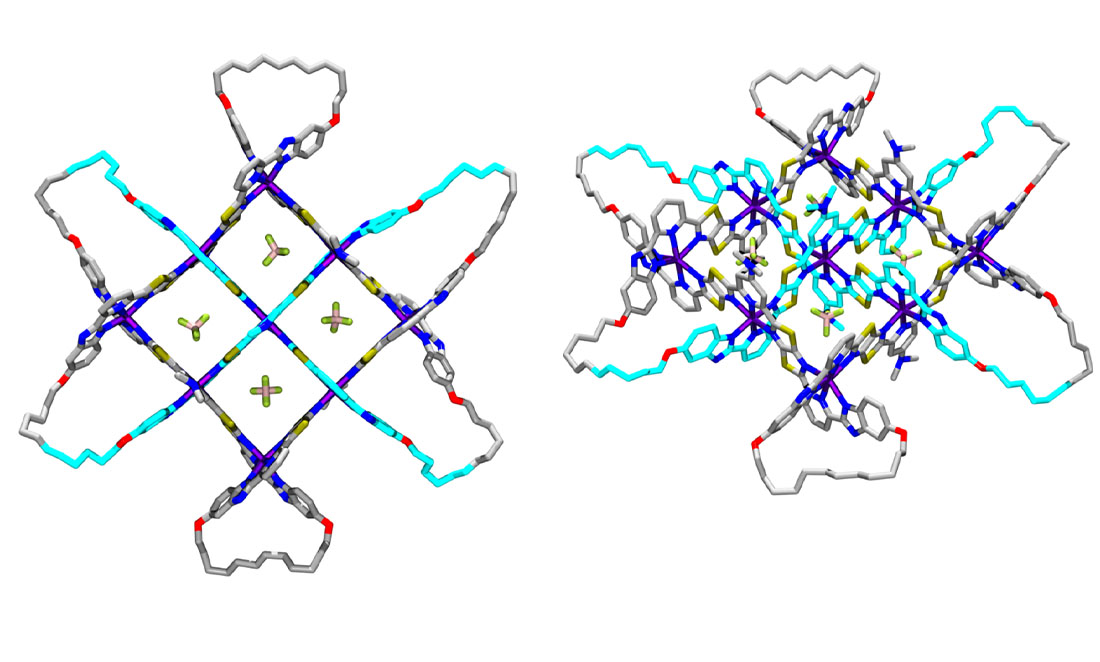
Metals make the core of a molecular knot. Iron or zinc atoms attract oppositely charged carbon molecules called ligands.
These ligands need to be created specifically for the knot.
For a seven-fold knot, they need three attachment points for metals. They also need to be flexible enough to weave but strong enough to stay together.
These ligands are woven through each other, then tied up using a process called olefin metathesis, which won the 2005 Nobel Prize in Chemistry.
Tying things up
If tying this knot sounds tricky, imagine doing it blindfolded.
Researchers can only add different chemicals during each step and can’t check these knots until the end.
They use chromatography to view the finished product. This figures out what chemicals are made of by measuring the light they absorb.
“We can use these knots to get a better understanding of how biological systems form, but also for building new materials. We don’t just want to understand these interactions, we want to make new things with them,” says George.
These knots could be used to make new organic materials, like new proteins and new chemical reactions for industrial chemistry.
While none of the scientists are going on to conquer Asia, they are trying to build more complex knots.









