Bog-standard batteries consist of electrochemical cells sandwiched together.
A single electrochemical cell may have a positive plate (cathode), a negative plate (anode) and an electrolyte.
Many car batteries are lead-acid batteries. This means the positive plate is a lead oxide, the negative plate is pure lead and the electrolyte is a mixture of sulfuric acid and water.
Electricity is formed from the flow of electrons and negatively charged particles that move from the anode to the cathode via a bridge.
Meanwhile, the electrolyte reacts with the plates, forming lead sulfate and water to maintain the difference in charge.
Electrons can’t just travel directly to the cathode – they have a big detour.
They must first pass through resistors that regulate the flow of electrons and transformers that convert electrons into other usable forms of energy.
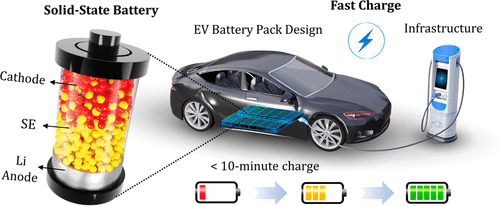
Credit: ACS Energy Lett. 2021, 6, 10, 3734-3749
WHAT IS A SOLID-STATE BATTERY?
Many of our current electrolytes are liquid-based.
This makes them heavy, flammable, corrosive and toxic.
Solid-state batteries remove that corrosive electrolyte soup of sulfuric acid and water and swap it for a block of glass, ceramic or polymer.
Now the ceramic isn’t an electrolyte converting the plate metals to maintain the charge difference.
Instead, the ceramic is full of tiny holes only large enough for the metal ions to move through in one direction.
So when the battery is recharged, the ions move from the anode to the cathode.
This means the battery can store more energy into a smaller size. This is why solid-state batteries are already finding use in small electronics like watches and pacemakers.
THE FUTURE IS SOLID
If solid-state batteries are so much better, why don’t we use them everywhere?
One big reason, common to all technological advancement, is that the old stuff was here first.
There are massive infrastructures built around the production of lead-acid batteries. These batteries still hold 42% of Australia’s battery market share.
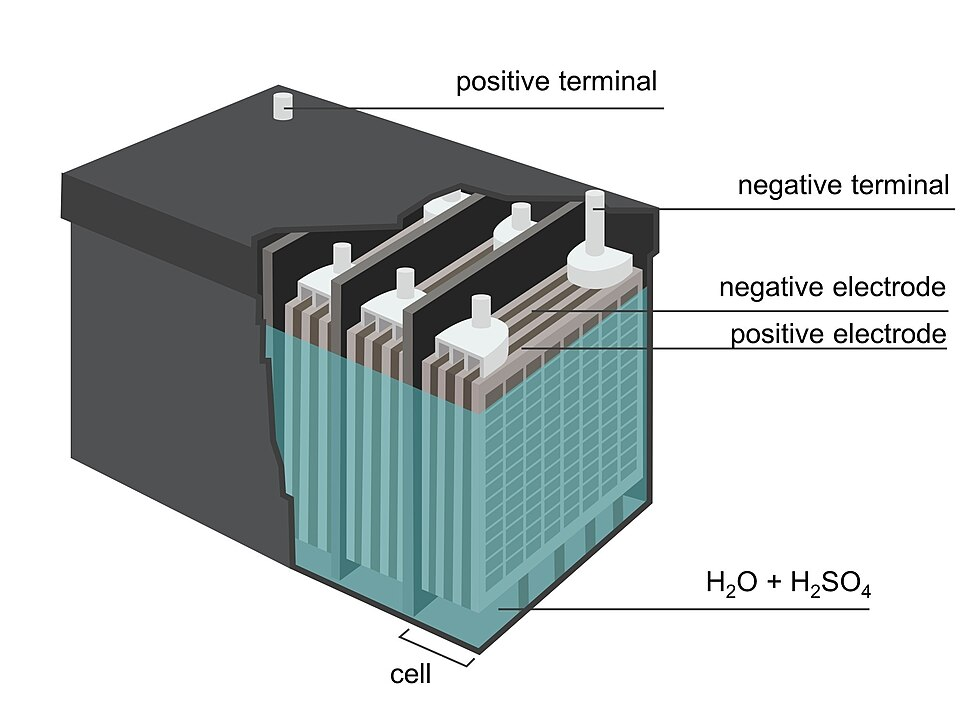
Credit: Paula Marengo
But the biggest technological reason is that solid-state batteries may experience problems with dendrites.
Over time, the anode will move through the solid electrolyte to physically reach the cathode. This causes hazards and degrades battery performance.
Despite these issues, solid-state batteries hold more charge for less weight. They also recharge much faster than traditional batteries.
That’s why Australian companies like Li-S are developing large solid-state batteries.
Last year, Perth company Altech Batteries began a solid-state battery trial in Germany.
This is just the beginning of a long journey for solid-state batteries.









