Batteries are a big deal for our electronic era and our renewable future.
Australia’s investing tens of millions in battery technology. The aim? Discovering safer and better batteries.
But what exactly makes a ‘battery’ and how do they work? Spoiler, there’s a lot of chemistry involved.

GIPHY
GO WITH THE FLOW
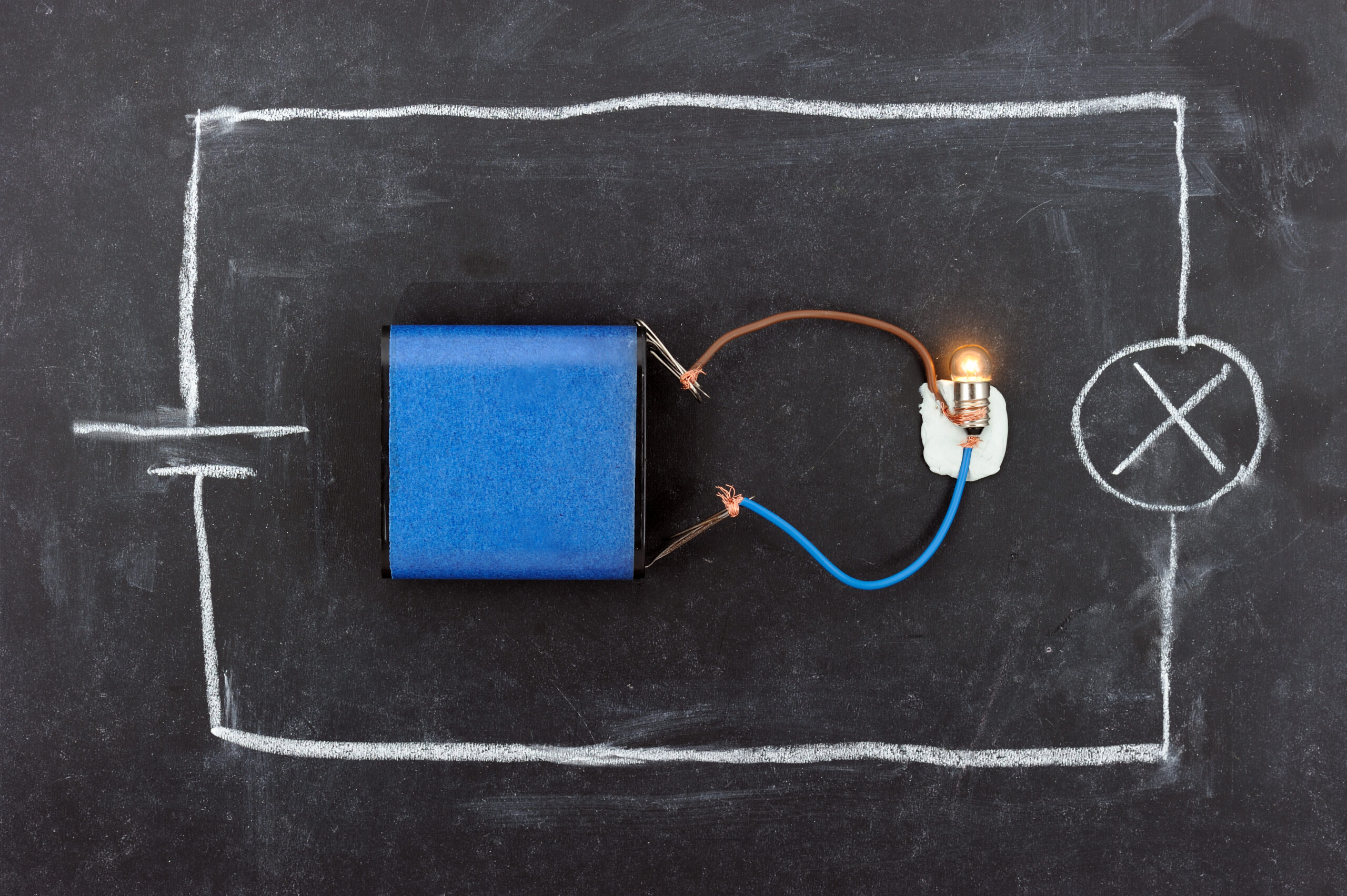
Batteries have a few basic components:
- Two electrodes: The positively charged ‘anode’ and the negatively charged ‘cathode’.
- An electrolyte: A liquid or gel that transports charged atoms – called ‘ions’ – between the electrodes. As electricity flows from the cathode to the anode, ions in the electrolyte move in the opposite direction, balancing the chemical energy.
- A separator: This is a porous material soaked in the electrolyte to enable ions to move through it while keeping the electrodes separated.
So essentially, a battery is a long, controlled chemical reaction. It generates power through a ‘redox reaction‘.
Once the reaction’s complete, the battery is dead and you need to recharge it or throw it away.
But what exactly is a ‘redox reaction’? To understand this, we need some fundamental chemistry.
RULES OF ATTRACTION
Atoms are the fundamental building blocks of chemistry. They’re made up of protons, neutrons and electrons.
Electrons are negatively charged, so an atom with more electrons than protons is considered ‘negative’.
In chemistry (and some romances!), opposites attract.
Electrons will often move from a negatively charged atom to a positively charged atom to balance the charges.
So how does a battery actually make electronics work?
HOW YOUR PHONE COMES ALIVE
When you insert a battery into a device – like your phone – the electrons at the cathode need to travel through a circuit to reach the anode.
The device you’re powering is part of the circuit. And it has its own chemicals that change properties when they receive electrons.
To be specific, the battery’s electrons flow into your phone, where silicon transistors rapidly switch on and off.
These switches create predictable patterns called ‘logic gates‘. These patterns become increasingly complex until your phone’s software emerges from the patterns.
Elsewhere, the battery’s electrons make it to magnetic plates that turn electricity into air vibrations. This creates sound on your phone speakers.
More electrons flow into crystals of gallium phosphide and gallium nitride that sit within your phone’s screen.
When these molecules gain electrons, they jump up in energy, releasing coloured light in response.
So there we have it! Mystery of the batteries unraveled!
Batteries have electrons that flow into chemicals within your phone (or any other electrical device), causing a series of awesome chemical reactions that make them come to life!

GIPHY