A team of US researchers have identified the brain circuits behind the mysterious placebo effect in mice, and they hope their research may inform the development of newer, safer pain-relieving drugs.
WHAT IS THE PLACEBO EFFECT?
Literally meaning “I please” in Latin, a placebo describes a substance or treatment with no actual therapeutic value – like a sugar pill or a saline injection.
Placebos are used in clinical trials as a baseline to compare with actual medical treatments – but there’s a problem. Enter the placebo effect.
The placebo effect describes a common phenomenon where believing that a medicine will be effective can help reduce symptoms like pain or nausea, even if the medication itself doesn’t actually work.
This effect is not simply a trick of the mind. When your brain anticipates pain relief, it will produce and release its own pain-relieving molecules called opioids.
According to Dr Grégory Scherrer, a professor in the Department of Cell Biology and Physiology at the University of North Carolina (UNC) and a co-author of the new study, the pain-relieving power of the placebo effect is comparable to a small dose of morphine – the opioid prescribed for severe pain in hospitals.
The placebo effect is so powerful it can seriously hamper the results of clinical trials because the effect of the real drug may be no greater than the effect of the placebo.
But despite being a widely known phenomenon, observed and reported since the early 1800s, the brain mechanisms that enable it have always been hazy – until now.
WHAT HAVE THEY FOUND?
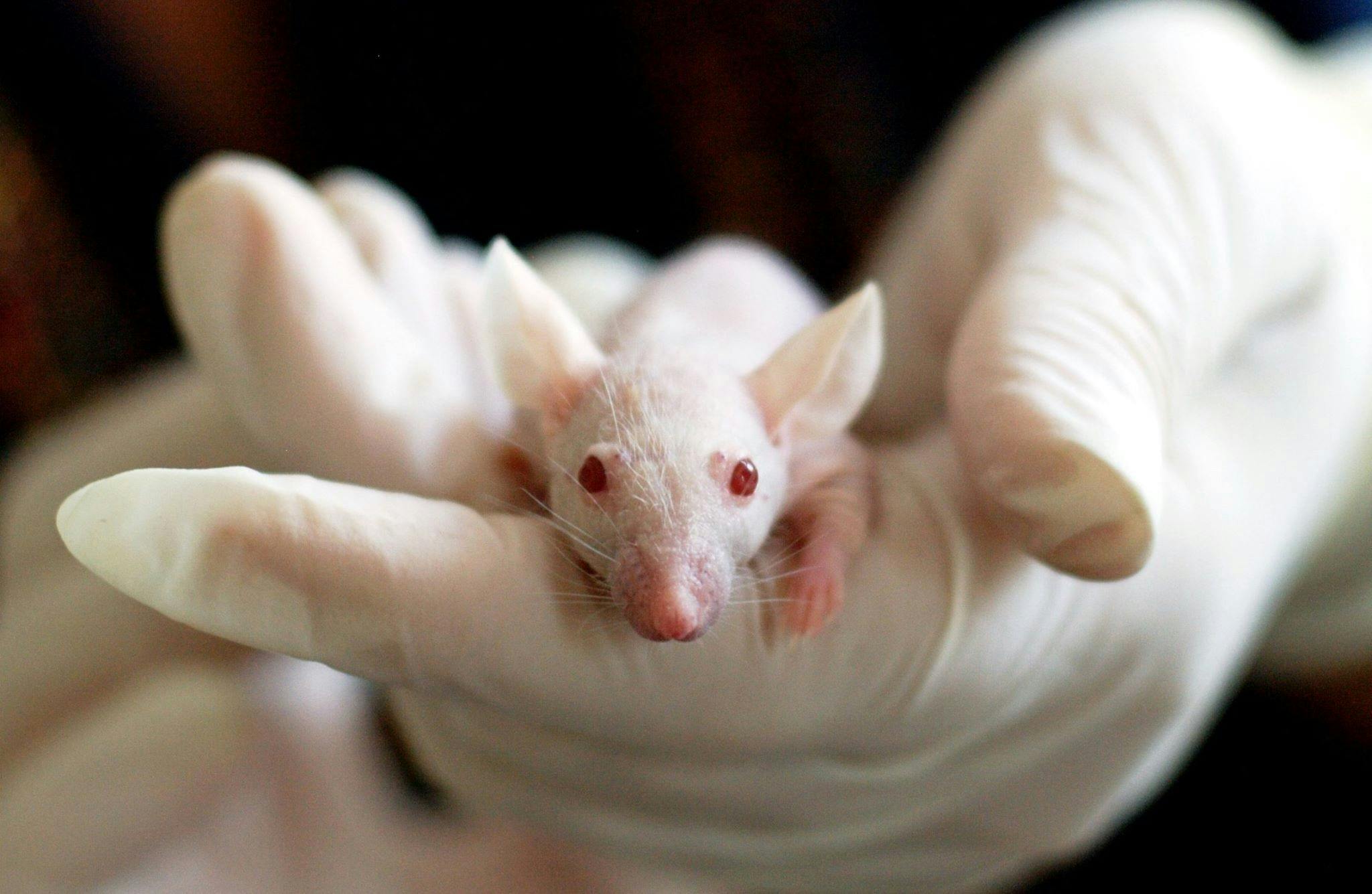
The team at UNC induced the placebo effect in lab mice by training them to associate two separate chambers with different floor temperatures – one at a mild 30°C, the other a painful 48°C. This trained the mice to associate the cooler chamber with pain relief.
Then the chambers were both set painfully hot. The mice that had been conditioned would show a clear preference for the chamber that had previously provided relief. What’s more, those mice would exhibit fewer behaviours associated with pain when in that chamber, like paw licking, rearing and jumping.
Satisfied those mice were under the influence of the placebo effect, the team used calcium imaging to identify the brain pathways that were firing. They found that the placebo effect was associated with a boost in the activity of the neurons that travel from the anterior cingulate cortex (ACC) – a region at the front of the brain known to play a role in emotion, attention and mood – to the pontine nucleus, a collection of neurons in the brainstem that feeds into the cerebellum, a brain region involved in movement and motor control.
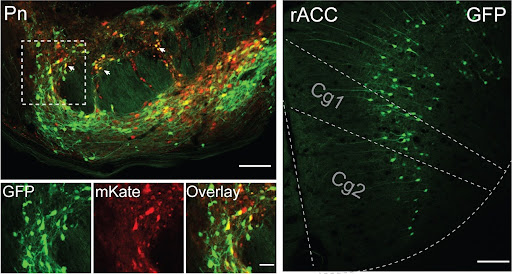
While the ACC was known to play a role in the placebo effect, this relationship with the pontine nucleus and the cerebellum was, according to the team, “a complete surprise”.
In follow-up studies, the team found a high concentration of opioid receptors – receptors that bind to pain-relieving molecules – in the pontine nucleus, cementing the idea that this area plays a role in pain. They had their smoking gun.
The cerebellum, into which the pontine nucleus feeds, is what Grégory calls a “big learning machine”, which helps to solidify memories associated with movement.
“The first time you touch a cactus, you’ll remember it your whole life,” he muses.
WHAT’S THE USE?
Grégory hopes these results will do more than simply improve our understanding of the brain.
“We now have another circuit that we can target for pain relief in patients with chronic pain,” he says. “We think we could possibly develop a completely new generation of painkillers.”
But he is cautious about drawing broad conclusions from early animal studies. There’s a wide gulf between the brain of a mouse and the brain of a human, and animal studies are not always reliable predictors of success in human clinical trials.
Some medicines have corresponding effects across species like penicillin, which can treat staph infections in mice and humans.
But other drugs have vastly different impacts across species like thalidomide, which causes major birth defects in humans but has no effect on rats and mice.
The next step is to identify whether the same pathway is involved in the placebo effect in humans, and Grégory is hopeful.
“The pain system is very well conserved [across the two species] because mice, like humans, have to be able to make predictions about pain to survive.”